Thermally Activated Delayed Fluorescence Is a Key New Technology for OLED Displays
Thermally Activated Delayed Fluorescence Is a Key New Technology for OLED Displays
Thermally Activated Delayed Fluorescence Is a Key New Technology for OLED Displays
TADF emitters are poised to contribute to the next material-driven advancement of the OLED industry, making OLEDs ideal for even more applications.
by Daniel Volz
In the last few years, the development of new materials has had a significant impact on the advancement of organic light-emitting diodes (OLEDs). Thanks to these advanced materials, OLED displays are now being used in smartwatches, smartphones, and TVs. However, there is still room for improvement in areas like display resolution and energy efficiency. Thermally activated delayed fluorescence (TADF) is a relatively new technology that has been developed to tackle these issues. This article describes the crucial role of emitter materials in OLED technology, and introduces the TADF concept.
OLEDs were first developed in the 1990s,1 but began to be used in commercial products just several years ago. Inside an OLED, the main properties are dictated by the so-called emitter, a molecule that can make direct use of the energy provided by the electric current to generate visible light. A simplified diagram of an OLED appears in Fig. 1.
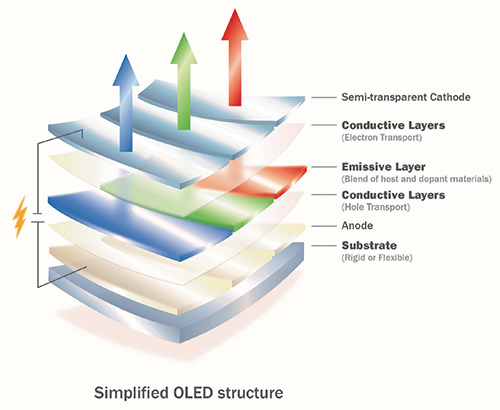
Fig. 1: OLEDs consist of multiple thin, organic layers. While this simplified structure shows a total of six layers including the substrate, commercial OLEDs sometimes
consist of many more layers – as many as 15 or more. The emitter or dopant materials are in the emissive layer.
Role of the Emitter in OLEDs
Three main principles are used to convert electrical energy into light: fluorescence, phosphorescence, and thermally activated delayed fluorescence (TADF). In the earliest OLEDs, fluorescent materials (FLUO) were used.3 Around 1998, it was found that phosphorescent materials (PHOS) could also be used in OLEDs, which led to a significant increase in efficiency.4,5 Recently, TADF materials have also been found to be suitable for very efficient OLED devices.6–8 TADF can be as efficient as phosphorescence, with up to 100% internal quantum efficiency.
The impact of using different emitter materials in commercial products can be seen in Fig. 2, where selected key performance indicators are plotted for various generations of Samsung’s Galaxy smartphones. The initial commercialization of OLED displays was possible only after the development of phosphorescent red emitters with suitable stability and efficiency.4
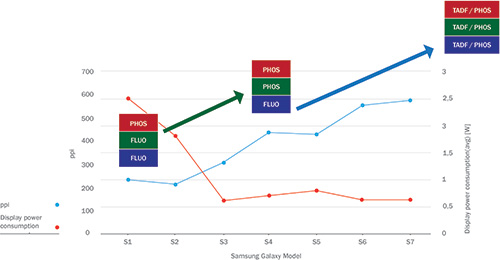
Fig. 2: The display resolution (in pixels per in. or ppi) and average power consumption (in watts) of several generations of Samsung Galaxy smartphones featuring OLED displays shows that significant improvement correlates to the use of new generations of materials. (Credit: Display power management data and ppi are from the DisplayMate article series on the Samsung Galaxy smartphones. Displaymate.com/mobile.html.)
Consequently, the first-generation Samsung phone, the S1, featured fluorescent materials for green and blue pixels, while red pixels contained phosphorescent materials. After the introduction of green phosphorescent materials, average power consumption dropped from 2.5 to well below 1 watt. The display’s resolution was also doubled to roughly 400 pixels per inch. Using the more efficient phosphorescent green materials allowed display manufacturers to significantly decrease the size of green pixels, which allowed for an increase in resolution.
The main differences between fluorescence, phosphorescence, and TADF are summarized in Table 1 and Fig. 3. For fundamental physical reasons, FLUO is less efficient than PHOS or TADF. The differences are caused by quantum-mechanical effects that lead to the formation of two different kinds of excited states when the electrical energy is being transferred to the emitter molecule: so-called singlet and triplet excitons. Because of quantum statistics, singlets and triplets are formed in a ratio of 1:3 upon recombination of electrically injected electrons and holes.7–9 FLUO is only capable of using singlet excitons, meaning that only 25% of the formed excitons will generate light, while 75% of the electric energy going into the OLED device is essentially lost.
Table 1: The major differences among the main emission principles (FLUO, PHOS, and TADF) used in OLEDs are shown in terms of performance and features.
| |
Performance |
Key feature |
| FLUO |
25% of the energy can be used for light generation |
Generally used for blue and often green pixels.
Focus on efficient emission from the S1 to S0 level to use singlet exciton emission. |
| PHOS |
100% of the energy can be used for light generation |
Generally used for red pixels and in some cases also in green pixels. Blue phosphorescent emitters do not show enough stability.
Heavy-metal-based materials that feature large spin-orbit coupling to harvest both singlet and triplet excitons. Metals can be iridium, platinum, or osmium, for example. |
| TADF |
100% of the energy can be used for light generation |
Comparable efficiencies to PHOS but a higher stability is expected.
Materials feature carefully adjusted energy levels S1 and T1, to allow for a small ΔE(S-T) to harvest both singlet and triplet excitons. |
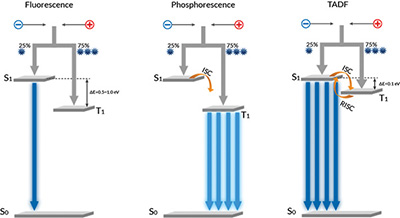
Fig. 3: Emission principles used in OLEDs include: fluorescence (FLUO), which often relates to the first emitter material generation; phosphorescence (PHOS),
which is the second material generation; and TADF, which marks the latest conceptual development.
PHOS and TADF employ two different strategies to generate photons from both singlet and triplet excitons. These strategies enable 100% efficiency:
• PHOS emitter materials employ rare heavy metals such as platinum and iridium to enable a quantum-mechanical phenomenon called spin-orbit coupling, which increases the radiative rate of triplet excitons. The main downside of this approach is the availability (and price) of such elements. Even elements such as uranium or rare earth elements (such as lanthanum and gadolinium) are more abundant than iridium.2
• TADF emitters, on the other hand, are metal-free molecules. A special molecular design principle evens out the energetic differences that normally are found between singlets and triplets. In fact, the energetic differences are so small that the thermal energy at normal environmental temperatures is enough to help excitons move from the triplet to the singlet state, where they can then be transformed to visible light. Using this process, every exciton in the device can be used to emit light, which corresponds to 100% internal quantum
efficiency and is comparable to phosphorescent emitters.
Electroluminescence mechanisms aside, FLUO, PHOS, and TADF emitters are synthesized using well-established chemical techniques similar to those employed for the large-scale production of pharmaceuticals such as aspirin, as well as for fertilizers and plastics.
Closing the Blue Gap in Displays
Aside from sustainability concerns, which stem from the fact that extremely rare elements such as iridium are required to make PHOS materials, there is also a technical issue that favors TADF over PHOS materials: the blue gap, which denotes the current trade-off between the efficiency and the stability of blue emitters. Figure 2 indicates that as of today, blue pixels of commercial products contain FLUO materials, even though they are less efficient. The reason for this is that – even after almost 20 years of industrial and academic research in the field of PHOS emitters – science has failed to produce a blue PHOS material that combines efficiency, stability, and a proper color point. If a blue emitter were to show high efficiency and long lifetime, as TADF is promising to do, this would create great opportunities to develop better products with even lower power consumption and better resolution. To date, it has been demonstrated that TADF emitters can deliver an excellent blue color point, while methods to achieve improved efficiency and stability are still being developed.
Figures 4 and 5 indicate the impact of the blue gap in existing blue fluorescent emitters. All OLED displays currently require relatively large blue pixel areas to reach enough brightness in the display. In smartphones, with red, green, and blue pixels, the blue pixel makes up 52% of the total area. Having TADF or PHOS pixels with a much higher efficiency would enable the display manufacturers to make smaller blue pixels to yield the same amount of light, which would pave the way again to increased resolution. Apart from a better display, customers would also benefit from a longer battery life for their mobile device. Battery life is closely connected to the power consumption of the display.
OLED TVs have a complex stack architecture, essentially making white light from red, green, and blue emitting layers and then using color filters to separate the colors again for the different pixels. Having a more efficient blue, which again makes up about 50% of the display area, would effectively reduce the power consumption of the TV by switching to a less complex stack design (such as in Fig. 4), which could potentially reduce manufacturing costs.
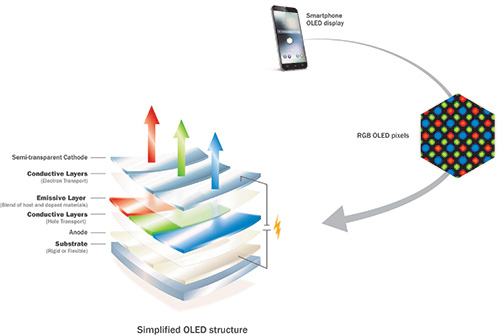
Fig. 4: This simplified OLED structure is the type generally used in smartphone displays. The light emitted by the blue, red, and green pixels directly generates the displayed image.
Currently, the blue pixel area is on the order of 52% of the total display area.
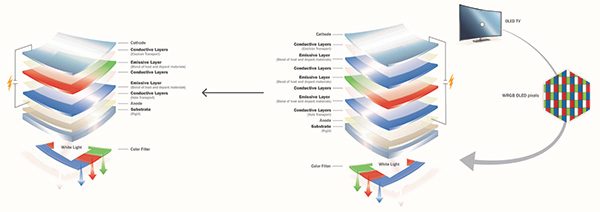
Fig. 5: This simplified OLED structure is the type commonly used in TV displays. For such applications, complex architectures are used to create white light, including color filters to create the image.
Currently, the surface area of blue pixels is on the order of more than 50%.
TADF Is Catching Up Quickly
Conceptually, donor and acceptor groups such as the ones shown in Fig. 6 can be connected in many different ways, as is illustrated in Fig. 7, which provides several recent examples for actual TADF molecules.
During the last two years we have witnessed major improvements in TADF materials, which now can surpass (blue) or reach similar performance (red and green) as conventional PHOS materials in terms of color point, efficiency, and stability. A main driving force behind this progress was the development of suitable screening algorithms for molecular design, which make use of density functional theory (DFT) calculations (Fig. 6). These computational tools drastically increased the efficiency of material development by allowing only promising, highly efficient TADF materials to be synthesized. This leads to extremely short material development cycles with a very steep learning curve per cycle.
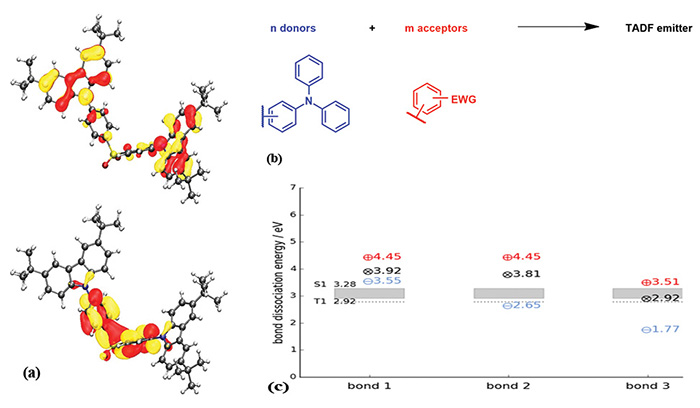
Fig. 6: Density functional theory (DFT) calculations are one cornerstone of the development of TADF materials (a). It is possible to use the computational prognosis, based on empirical results,
as a pre-screening tool to avoid bad TADF emitters. This methodology drastically accelerates material development. Conceptually, TADF molecules consist of donors and acceptors
covalently bonded (b). Apart from the prediction of TADF properties, molecular issues can also be seen in DFT calculations.
Weak bonds, for example, can be identified by calculating the bond dissociation energy (c).
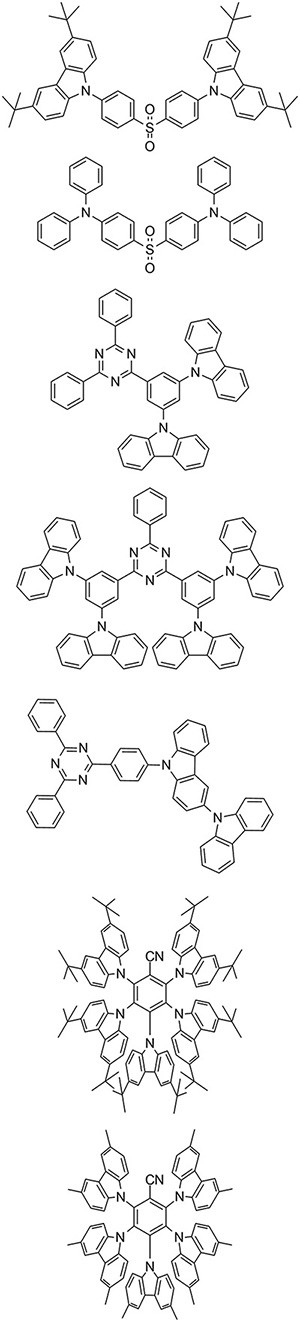
Fig. 7: These selected blue TADF emitters were recently published by researchers Lian Duan, Chihaya Adachi, and Jun Yeob Lee.10–12
Results based on this development approach were recently published by our team at CYNORA. We reported several blue materials, among them a blue emitter reaching 14% external quantum efficiency (EQE) at 500 nits and a lifetime to 80% of the initial luminance (LT 80) of 420 h at 500 nits starting luminance.13 This material featured an emission maximum below 480 nm. Properties of a recently developed material are shown in Table 2 and Figs. 8 and 9. The electro-optic JVL characteristics of this material in a simple device architecture, using the literature-known host mCBP14 (short for 4,40-bis(3-methyl-carbazol-9- yl)-2,20-biphenyl), demonstrate about 4.2 volts at 500 nits. The so-called blue index, which can be calculated by dividing the current efficiency in cd A–1 and the CIEy color coordinate (for CIE coordinates, see Fig. 10), approaches 80 at 500 nits in a bottom-emitting device.
Ir(dmp)3, short for iridium (III) tris[3-(2,6-dimethylphenyl)-7-methylimidazo[1,2-f] phenanthridine], currently represents (in the literature) the most stable blue iridium emitter with a relatively blue color point (CIE 0.15 / 0.31, see Fig. 10 for definition) and decent efficiency of 8% EQE at 1,000-nits luminance in a conventional OLED architecture. In a recent study by Forrest and co-workers,15 the basic performance was reported in the same host used to obtain the data shown in Table 2. The LT80 at 500 nits was estimated from the values given in the publication. Even using a simple, non-optimized screening architecture and normal R&D-grade purity, the stability LT80 at this starting luminance is on the order of 100 hours, which is in the range of the best phosphorescent materials when also considering the better color and the higher efficiency15 (Table 2). Considering that phosphorescent materials have been under investigation since 1997,4 while TADF has been studied only since 2011,6 these results demonstrate a significant development curve for TADF.
Table 2: This table shows the device performance of the CYNORA material (TADF) vs. Ir(dmp)3, currently the most stable and efficient blue phosphorescent emitter,15 in 13 w-% mCBP (PHOS).
| |
CIE (1000 nits) |
EQE (1000 nits) |
LT80 (500 nits) |
| TADF |
(0.17, 0.27) |
12% |
94 |
| PHOS |
(0.16, 0.31) |
8% |
ca. 100 |
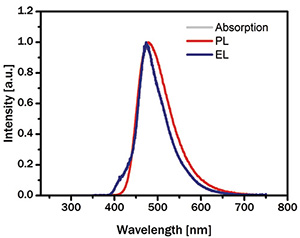
Fig. 8: Above is a comparison between the photoluminescence (PL) of a mid-blue TADF emitter and its electroluminescence (EL) performance in a bottom-emitting OLED.
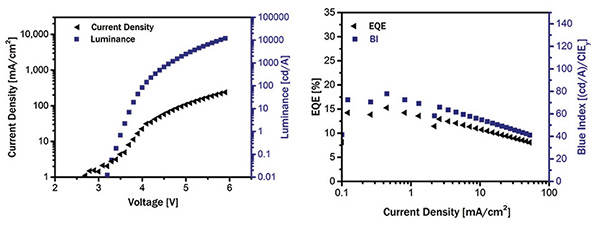
Fig. 9: Above is shown the performance of a recent deep-blue TADF OLED material, with voltage at left and luminance at right.
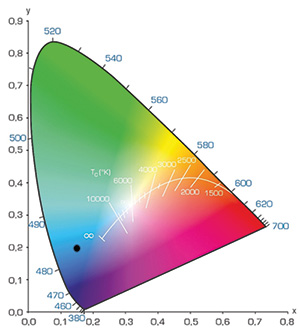
Fig. 10: The color of OLEDs is measured in the CIE 1931 color coordinate system. To satisfy the color expectations of the display industry,
CIEy values below 0.20 – ideally even around CIEy 0.10 – are required. With current TADF materials, CIEy values in the order of 0.15 can be reached.
What to Expect from TADF Technology in the Future
By 2015, CYNORA and others7 had established that it was possible to realize a favorable deep-blue color point with TADF material technology. Recently, great progress toward more stable blue TADF devices has been made, demonstrating materials with emission below 480 nm, 14% EQE, and lifetime values of LT80 at 420 hours (measured at 500 nits brightness).13 Within a relatively short period of R&D, TADF emitters have now reached a performance similar to PHOS emitters with a blue color point. The cornerstone of these successes was a fast translation of quantum-chemical predictions from DFT-calculations into material design and a continuous improvement of the underlying theory, which led to improved materials in each learning cycle. Following this trend, blue TADF technology should reach market readiness by the end of 2017, according to CYNORA’s roadmap.
Further improvement can be expected through the realization of more sophisticated stack architectures. For example, mCBP, a component used in the aforementioned early-stage devices with CYNORA’s material, is known to have several stability issues, potentially limiting the stability of these OLEDs.16 This indicates that using other, more stable hosts will lead to even longer lifetimes.
Nevertheless, it is also clear that the basic stability of both the materials and the stack architectures still need to be improved fundamentally. Looking back at a very steep learning curve displayed by research-driven companies such as CYNORA, as well as great academic progress10,17,,18 this necessary advancement seems achievable in a short amount of time. TADF will soon contribute to the next material-driven advancement of the OLED industry, making OLEDs ready for even more applications soon.
References
1C. W. Tang, S. Van Slyke, “Organic electroluminescent diodes,” Appl. Phys. Lett. 51, 913 (1987).
2D. Volz, et al., “From iridium and platinum to copper and carbon: new avenues for more sustainability in organic light-emitting diodes,” Green Chem. 17, 1988–2011 (2015).
3S. Van Slyke, C. H. Chen, C. W. Tang, “Organic electroluminescent devices with improved stability,” Appl. Phys. Lett. 69, 2160 (1996).
4M. A. Baldo, et al., “Highly efficient phosphorescent emission from organic electroluminescent devices,” Nature 395, 151–154 (1998).
5M. A. Baldo, S. Lamansky, P. E. Burrows, M. E. Thompson, S. R. Forrest, “Very high-efficiency green organic light-emitting devices based on electrophosphorescence,” Appl. Phys. Lett. 75, 4 (1999).
6A. Endo, et al., “Efficient up-conversion of triplet excitons into a singlet state and its application for organic light emitting diodes,” Appl. Phys. Lett. 98, 083302 (2011).
7L. Bergmann, D. M. Zink, S. Bräse, T. Baumann, D. Volz, “Metal–Organic and Organic TADF-Materials: Status, Challenges and Characterization,” Top. Curr. Chem. 374, 22 (2016).
8D. Volz, “Review of organic light-emitting diodes with thermally activated delayed fluorescence emitters for energy-efficient sustainable light sources and displays,” J. Photonics Energy 6, 020901 (2016).
9H. Yersin, A. F. Rausch, R. Czerwieniec, T. Hofbeck, T. Fischer, “The triplet state of organo-transition metal compounds. Triplet harvesting and
singlet harvesting for efficient OLEDs,” Coord. Chem. Rev. 255, 2622–2652 (2011).
10D. Zhang, M. Cai, Y. Zhang, D. Zhang, L. Duan, “Sterically shielded blue thermally activated delayed fluorescence emitters with improved efficiency and stability,” Mater. Horiz. 3, 145–151 (2016).
11H. Tanaka, K. Shizu, H. Miyazaki, C. Adachi, “Efficient green thermally activated delayed fluorescence (TADF) from a phenoxazine–triphenyltriazine (PXZ–TRZ) derivative,” Chem. Commun. 48, 11392 (2012).
12S. Youn Lee, T. Yasuda, H. Nomura, C. Adachi, “High-efficiency organic light-emitting diodes utilizing thermally activated delayed fluorescence from triazine-based donor–acceptor hybrid molecules,” Appl. Phys. Lett. 101, 093306 (2012).
13CYNORA GmbH, October 2016, press release, www.cynora.com
14G. Li, T. Fleetham, E. Turner, X. C. Hang, J. Li, “Highly efficient and stable narrow-band phosphorescent emitters for OLED applications,” Adv. Opt. Mater. 3, 390–397 (2015).
15Y. Zhang, J. Lee, S. R. Forrest, “Tenfold increase in the lifetime of blue phosphorescent organic light-emitting diodes,” Nat. Commun. 5, 1–7 (2014).
16K. P. Klubek, C. W. Tang, L. J. Rothberg, “Investigation of blue phosphorescent organic light-emitting diode host and
dopant stability,” Org. Electron. 15, 1312–1316 (2014).
17J. W. Sun, K. Kim, K. C. Moon, J. H. Lee, J. Kim, “A highly efficient sky-blue fluorescent organic light emitting diode based on mixed cohost system for thermally activated delayed fluorescence emitter (2CzPN),” ACS Appl. Mater. Interfaces 8, 9806–9810 (2016).
18H. Nakanotani, K. Masui, J. Nishide, T. Shibata, C. Adachi, “Promising operational stability of high-efficiency organic light-emitting diodes
based on thermally activated delayed fluorescence,” Sci. Rep. 3, 2127 (2013). •
Daniel Volz studied chemistry at the Karlsruhe Institute of Technology and Karlsruhe School of Optics and Photonics (KSOP) in Germany. He received his Ph.D. (summa cum laude) in 2014. He joined CYNORA’s R&D department in 2009, where he served in various roles. Currently, he serves as Material Strategist and is responsible for the development of CYNORA’s material portfolio. Volz was awarded with the Carl Roth Prize for sustainable use of chemicals in 2014 as well as the Green Photonics Award and the Klaus Tschira Award for Achievements in the Public Understanding of Science in 2015. He can be reached at info@cynora.com.